
TOKYO, Feb 3, 2026 - (JCN Newswire) - Olympus Corporation (Olympus), a global MedTech company committed to making people’s lives healthier, safer and more fulfilling, today announced publication of the EAGLE Trial1 — a multicenter randomized controlled study evaluating the CADDIE™ device, the first cloud-based Computer-Aided Detection (CADe) application for real-time polyp detection during colonoscopy that is both FDA-cleared and CE-marked. The CADDIE software is the first solution introduced as part of the OLYSENSE™ Intelligent Endoscopy Ecosystem.
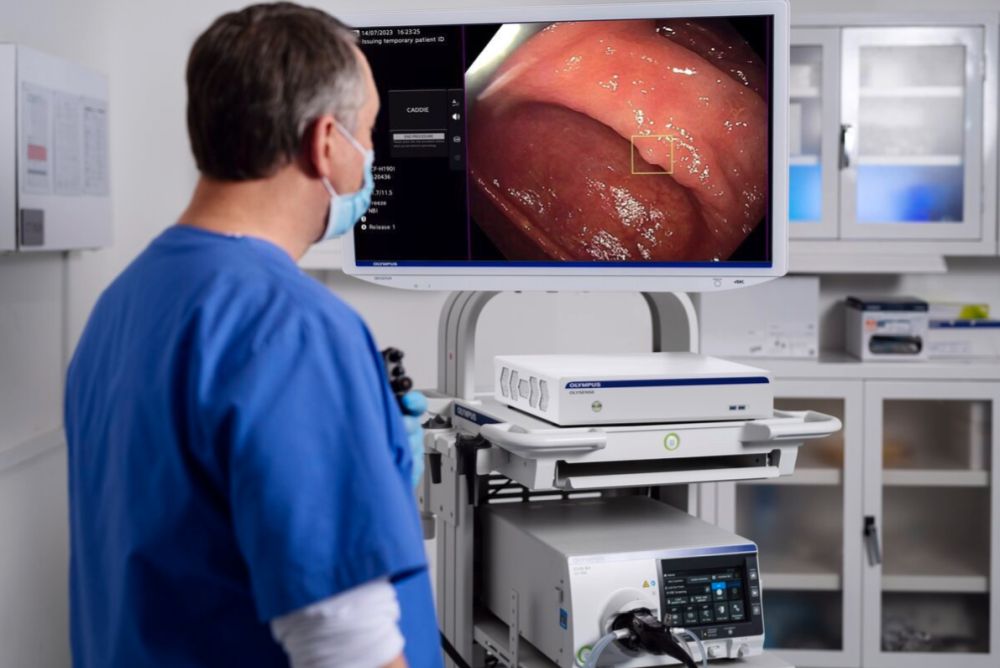
The trial indicates that cloud-deployed AI can help endoscopists detect the lesions that matter most in preventing progression to cancer2-5 — large adenomas, particularly those flat in morphology, and sessile serrated lesions (SSLs) — without disrupting safety or workflow. The study has been published in npj Digital Medicine.1
EAGLE Trial Overview
The EAGLE (Evaluation of AI for detection of Gastrointestinal Lesions in Endoscopy) study was conducted across eight centers in four European countries6, and its primary analysis involved 841 patients and 22 endoscopists performing screening and surveillance colonoscopies. Patients were randomized to standard colonoscopy or CADDIE-assisted colonoscopy.
Key Findings
- Improved detection of high-risk and hard-to-detect lesions
In screening and surveillance patients, use of the CADDIE™ application was associated in this study with 7.3% absolute increase in adenoma detection rate (ADR), compared to standard colonoscopy. Moreover, significant relative increases in lesions detected per colonoscopy were observed in this study for clinically relevant lesion subtypes: 93% for large (>10 mm) adenomas, 57% for non-polyploid adenomas and 230% for SSLs. - Feasible and efficient real-time cloud-based deployment
The system demonstrated real-time performance and operational efficiency across diverse testing environments.
Clinically Relevant by Design
The CADDIE™ application is trained on a dataset enriched in clinically relevant and hard-to-detect lesions, including flat sessile serrated lesions (SSLs) and large polyps (≥10 mm).
Lesions with sessile or flat morphology are difficult to detect and can harbor clinically relevant pathology. SSLs, in particular, are high-risk lesions whose detection is critical to reducing the risk of post-colonoscopy colorectal cancer3-4. The ability to reliably detect SSLs is increasingly viewed as a critical quality consideration in colonoscopy7. This study demonstrates increased detection of clinically relevant lesions and no increase in unnecessary resections, addressing some of the concerns raised in recent guidelines8-9.
Why Cloud Matters?
The CADDIE™ application leverages a cloud architecture that uses industry standard security controls. Cloud deployment offers hospitals flexibility, reducing reliance on hardware and enabling subscription-based procurement models. This approach can democratize access to advanced AI tools and lays the foundation for future AI applications in endoscopy.
Expert Perspectives
“This study marks a pivotal shift in the clinical translation of AI-assisted endoscopy,” said Rawen Kader, Principal Investigator of the EAGLE Trial and GI Researcher at University College London. “Cloud deployment can remove hardware barriers and give hospitals access to the latest AI innovations, which has the potential of improving detection of the lesions that matter most for reducing colorectal cancer risk.”
“The publication of EAGLE study in a high-impact journal like npj Digital Medicine is a pivotal moment for Olympus, supporting clinical adoption of the CADDIE™ device as an AI solution that can enhance detection of clinically relevant lesions without compromising safety or efficiency,” said Miquel Àngel García, Executive Vice President and General Manager, Gastrointestinal Business Unit, Olympus Corporation.
“The EAGLE trial demonstrates how cloud‑based AI can be translated into routine endoscopy at scale,” said Peter Mountney, CEO of Odin Vision and Vice President, AI Unit, Olympus Corporation. “By delivering AI in real time through the cloud, we can help accelerate innovation and enable hospitals around the world to benefit from our latest, evidence‑based technologies to support clinicians and enhance the quality of care.”
For complete access to the EAGLE Trial study, visit: www.nature.com/articles/s41746-025-02270-1
Fair Balance Statement
The gastroenterologist is responsible for reviewing CADDIE suspected polyp areas and confirming the presence or absence of a polyp based on their own medical judgment.
CADDIE is not intended to replace a full patient evaluation, nor is it intended to be relied upon to make a primary interpretation of endoscopic procedures, medical diagnosis, or recommendations of treatment/course of action for patients. The CADDIE computer-assisted detection device is limited for use with standard white-light endoscopy imaging only.
1. Kader R, Hassan C, Lanas Á, et al. A novel cloud-based artificial intelligence for real-time detection of colorectal neoplasia – a randomized controlled trial (EAGLE). npj Digit.l Med.. Published online December 26, 2025. https://doi.org/10.1038/s41746-025-02270-1
2. Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020;158(2):291-302. doi:10.1053/j.gastro.2019.08.059
3. Anderson JC, Hisey W, Mackenzie TA, et al. Clinically significant serrated polyp detection rates and risk for postcolonoscopy colorectal cancer: data from the New Hampshire Colonoscopy Registry. Gastrointest Endosc. 2022;96(2):310-317. doi:10.1016/j.gie.2022.03.001
4. Toledo DEFWMv, IJspeert JEG, Bossuyt PMM, et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: a population-based study. Lancet Gastroenterol Hepatol. 2022;7(8):747-754. doi:10.1016/s2468-1253(22)00090-5
5. Soetikno RM. Prevalence of Nonpolypoid (Flat and Depressed) Colorectal Neoplasms in Asymptomatic and Symptomatic Adults. JAMA. 2008;299(9):1027. doi:10.1001/jama.299.9.1027
6. Italy, Germany, Spain, Poland
7. Rex DK, Anderson JC, Butterly LF, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2024;100(3):352-381. doi:10.1016/j.gie.2024.04.2905
8. Bretthauer M, Ahmed J, Antonelli G, et al. Use of computer-assisted detection (CADe) colonoscopy in colorectal cancer screening and surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. Published online 2025:. doi:10.1055/a-2543-0370
9. Sultan S, Shung DL, Kolb JM, et al. AGA Living Clinical Practice Guideline on Computer-Aided Detection–Assisted Colonoscopy. Gastroenterology. 2025;168(4):691-700. doi:10.1053/j.gastro.2025.01.002
About Olympus
At Olympus, we are committed to Our Purpose of making people’s lives healthier, safer and more fulfilling. As a global medical technology company, we partner with healthcare professionals to provide innovative solutions and services for early detection, diagnosis and minimally invasive treatment, aiming to improve patient outcomes by elevating the standard of care in targeted disease states. For more than 100 years, Olympus has pursued a goal of contributing to society by producing products designed with the purpose of delivering optimal outcomes for its customers around the world. For more information, visit olympus-global.com and follow us on LinkedIn.
Media contact:
Mail: Global-Public_Relations@olympus.com
Olympus Corp [TYO: 7733] [ADR: OLYMY] [STU: OLY1] [FRA: OLYS] https://www.olympus-global.com
]]>Source: Olympus
Copyright 2026 JCN Newswire . All rights reserved.







