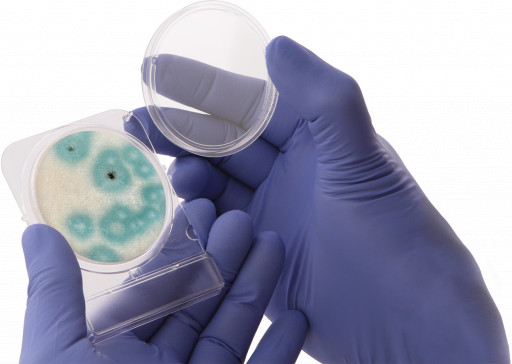
CompactDry Yeast Mold Rapid
SANTA MARIA, Calif. - June 16, 2021 - (Newswire.com)
Hardy Diagnostics is proud to announce that the popular media solution CompactDry Yeast Mold-Rapid has achieved a matrix extension validation for dried cannabis flower in the recent AOAC Emergency Response Validation program. This certification is an important step forward for cannabis testing programs focused on both quality and regulation compliance. The easy-to-use media plate offers long-term, room temperature storage and provides both qualitative and quantitative results to its users. The rapid format is validated to provide reliable results for cannabis in just 72 hours, protecting the quick turnaround times most labs require. Chromogens simplify the distinction between yeasts and molds for technicians, and the construction of the plate provides the right amount of headspace for colonies to form naturally. This helps keep the fungal structures intact and makes identification and subculture easier as well.
Cannabis plants have long been known to harbor potentially dangerous pathogenic molds. This has led most regulators to require some form of mold testing program from the certified cannabis testing labs in their jurisdiction. One of the challenges facing these labs has been the lack of validated, standardized scientific methods. The legal landscape for cannabis across the U.S. has resulted in a patchwork of solutions and approaches, making it more difficult for everyone to achieve compliance. AOAC INTERNATIONAL took an important first step in addressing this issue by creating the Cannabis Analytical Science Program, or CASP. AOAC has a history of bringing together government, industry, and academia to establish standard methods of analysis that ensure the safety and integrity of foods and other products that impact public health around the world. AOAC Research Institute created a matrix extension protocol for method developers to validate their yeast/mold detection assays with dried cannabis flower. By partnering with cannabis testing labs across Michigan, solutions were tested and proven against the standards set by AOAC.
Hardy Diagnostics is a proud partner of the cannabis industry and has been actively supporting the microbiology needs of cannabis labs and cultivation for over six years. We are proud to present the entire line of CompactDry product assays to the cannabis industry, with the first CompactDry product (Yeast Mold-Rapid) now PTM certified for dried cannabis flower. As our industry continues to grow, you can trust that the Hardy team is there for you.
Contact Person: Jessa Youngblood
Company Name: HARDY DIAGNOSTICS
Voice Phone Number: (805) 346-2766, Ext: 5653
Fax Number: (805) 346-8199
Email Address: YoungbloodJ@HardyDiagnostics.com
Website: www.HardyDiagnostics.com

Related Files
AOAC cert YMR cannabis.pdf
19ddf878a0dad1259f3916313e79.pdf
Press Release Service by Newswire.com
Original Source: Hardy Diagnostics Achieves AOAC PTM Validation for the CompactDry Yeast and Mold Rapid Media Plate With Dried Cannabis Flower