Rimsys, the leading provider of Regulatory Information Management (RIM) software for the medtech industry, today announced the release of Rimsys 5 at the MedTech Forum in Barcelona, Spain. The three-year-old cloud-based regulatory software, already used by 10 of the 30 largest medical device manufacturers globally, helps medtech companies strengthen compliance and bring products to market more quickly. The new version of the software includes a comprehensive regulatory submissions module and provides integrated, global regulatory intelligence through a partnership with Clarivate Cortellis. Additional new features include an updated and highly-flexible product hierarchy and the ability to link product, performance, and safety data across the organization through standardized integrations with PLM, eQMS, and ERP systems.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220504005139/en/
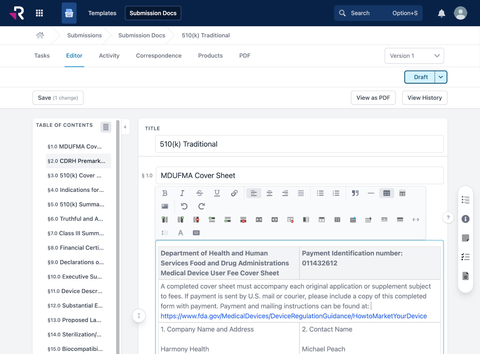
Rimsys new regulatory submissions module with government templates, collaborative content authoring, and compliant PDF generation with auto-created table-of-contents and appendices (Graphic: Business Wire)
“We took a really unique approach to regulatory submissions with Rimsys 5,” said Brad Ryba, Rimsys Co-founder and CTO. “With our solution, regulatory affairs teams can manage submission projects, collect documents and information, and directly author submission content in a single interface. Plus, customers have full access to their submissions archive without any additional costs. We believe this will really help regulatory teams improve their productivity and easily provide new functionality, strong integrations, and a great user experience.”
RIM software systems are becoming increasingly necessary as more and more countries adopt specific market entrance requirements and the global regulatory landscape becomes increasingly complex. This leaves medtech regulatory professionals on the front lines, responsible for correctly deciphering entrance requirements and coordinating the assembly of complex submission documents. A medtech RIM system can largely automate the administrative tasks that most RA professionals are spending at least 30% of their time on.
Rimsys 5 builds on an already established platform of product-centric regulatory tools, including standards management, expiration monitoring, market-specific sales status tracking, and more. With Version 5, regulatory teams can now:
- Access high-quality comprehensive regulatory information (powered by Clarivate Cortellis).
- Build content plans based on government templates, utilizing fully customizable submission templates for common market applications.
- Collaboratively author submission content.
- Track correspondence with health authorities.
- Manage submission projects.
- Auto-generate completed regulatory submissions.
In addition, Rimsys 5 introduces a completely flexible product hierarchy that allows medtech companies to fully mirror their product catalogs in their RIM system.
"Rimsys enables our submissions to be created, stored, and made easily accessible to everyone who needs them,” said a Regulatory Manager at a top 30 medical device company currently using the new features, “What really sets Rimsys apart is its ability to link data throughout the system, making it a highly functional and intuitive solution. RIMSYS allows us to create more streamlined business processes, and transparency across the entire company organization, which means we are able to bring our products to market much more quickly than ever before.”
All of these features combine to provide a full record of submission history directly linked to individual products, countries, and registrations - giving regulatory teams the tools they need to fully administer, track, and generate regulatory information for every product in every market.
About Rimsys
Rimsys is on a mission to bring regulatory order to the medtech industry. The Rimsys Regulatory Information Management (RIM) platform digitizes and automates regulatory activities, freeing teams from inefficient administrative work, and helping them confidently establish and secure global regulatory compliance. Unlike complex, color-coded spreadsheets, or expensive external consultants, Rimsys seamlessly centralizes all regulatory information, automates submission processes, and monitors relevant expirations, standards, and global regulations. Traditional approaches to regulatory affairs can’t keep pace with the growing complexity of the global landscape, and overburdened teams face increasing compliance risks. Rimsys streamlines all regulatory activities including registrations, essential principles, UDI, standards management, and regulatory intelligence in a single, integrated platform. Leading global medtech companies including Johnson & Johnson, Siemens Healthineers, and Omron rely on Rimsys to get new products to market more quickly, and reduce revenue risk of non-compliance, product recalls, and unexpected expirations. For more information, visit www.rimsys.io.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220504005139/en/
Contacts
Michael Peach, michael.peach@rimsys.io



