SOMAVAC Deep Tissue Negative Pressure Therapy
SOMAVAC\u00ae Deep Tissue Negative Pressure Therapy effectively removes fluids, obliterates dead space, and holds layers together. SOMAVAC\u00ae is FDA cleared for seroma prophylaxis.
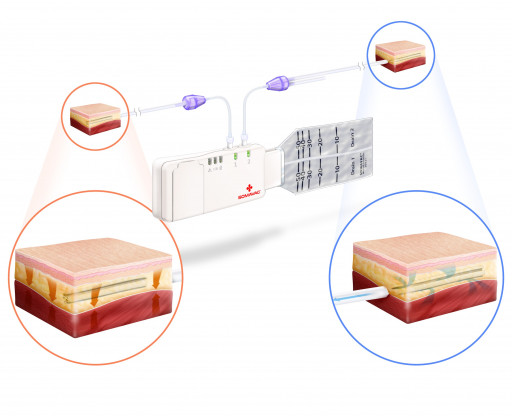
SOMAVAC® Medical Solutions, Inc. announces the issuance of a U.S. patent for the pioneering development of a deep tissue negative pressure therapy device (dtNPT) with the added benefit of closed-incision negative pressure wound therapy (ciNPWT). This patent is for the only comprehensive device that allows surgeons to optimize surgical outcomes with negative pressure therapy both inside the surgical site, as well as outside the incision, which creates a new medical device category and deepens the penetration of the $1.1B immediate U.S. market.
"For my cancer patients, post-operative complications and infections can delay adjuvant chemotherapy, which can break our protocols and lead to bad outcomes. With the SOMAVAC® dtNPT, my patients have the best chance of remaining on protocol, which leads to predictable good outcomes. The addition of ciNPWT will further mitigate the risks associated with wound complications in complex revisions and orthopaedic oncologic procedures," said Dr. Michael Neel, MD, Chief of the Division of Orthopaedics, St. Jude Children's Research Center, Memphis, TN.
Currently, the SOMAVAC® SVS is the only dtNPT device cleared by the FDA to prevent seromas. Most commonly used for mastectomies, breast reconstructions, hernia repairs, panniculectomies, abdominoplasties, tummy tucks and complex orthopedic reconstructions, the SOMAVAC® SVS applies dtNPT for continuous removal of fluids, obliterating dead space and helping hold tissues together, which may reduce the risk of seromas. Seromas can lead to complications like infection, delayed wound healing and revision surgery*.
With the addition of closed-incision NPWT to dtNPT, patients can experience wound healing from the inside and outside of the surgical site simultaneously. Closed-incision NPWT has been shown to reduce external contamination and hold incision edges together, thereby decreasing the incidence of wound dehiscence, surgical site infections (SSI) and other wound complications.*
"The SOMAVAC® SVS dtNPT is a patient-centered device that enhances every aspect of the patient experience with drains while driving drain care compliance. The addition of closed-incision NPWT will now add the established surgical site benefits of NPWT. This combination of dtNPT and closed-incision NPWT provides comprehensive surgical site care, creating an opportunity to minimize complications both inside and outside the surgical site," said Dr. Nathaniel Stoikes, MD, General Surgeon, Memphis, TN.
"This patent solidifies our intellectual property portfolio for complex surgeries. I am grateful to our outstanding team members and legal team for diligently pursuing this patent," said Josh Herwig, co-founder and CTO of SOMAVAC® Medical.
"This patent with the combination of closed-incision NPWT with dtNPT gives our team the latitude to advance our technology and pursue innovative solutions that will further enhance the clinical outcomes and improve the recovery experience," said Esra Roan, Ph.D., co-founder and CEO of SOMAVAC® Medical.
*data on file
Contact Information:
Sandra Saunders
Marketing
sandra.saunders@somavac.com
833.766.2822
Esra Roan
CEO
esra.roan@somavac.com
+18337662822
Press Release Service by Newswire.com
Original Source: SOMAVAC® Medical Solutions, Inc. Issued US Patent for combined dtNPT and ciNPWT Device




