 (PRLeap.com) Despite global economic and political crises, a London based group acquires 20% shares of BrainRepair UG, a spin-off of the Ruhr-University Bochum (RUB), Germany, that has developed a unique method based on own (autologous) cord blood stem cells to treat brain damage and cerebral palsy (CP) in newborns affecting 70,000 babies in the EU each year. BrainRepair UG has been granted the worldwide first 'Orphan Drug Designations' (ODD) by the European Commission / European Medicines Agency (EMA) and the Paediatric Investigation Plan (PIP) for the pivotal trial has been approved. The ODD guarantees market exclusivity in all EU member states for 12 years upon market authorisation. "We are particularly grateful to Olaf Bolwerk, Johan Nijboer, and Dr. Alexander van der Speld who masterminded the transaction to fund our way towards market authorisation", Prof. Dr. Arne Jensen stresses and continues, "All our personal, scientific, clinical, and philanthropic efforts serve the ultimate goal - to combat cerebral palsy, the most common disability in childhood - and stop CP in children!"
(PRLeap.com) Despite global economic and political crises, a London based group acquires 20% shares of BrainRepair UG, a spin-off of the Ruhr-University Bochum (RUB), Germany, that has developed a unique method based on own (autologous) cord blood stem cells to treat brain damage and cerebral palsy (CP) in newborns affecting 70,000 babies in the EU each year. BrainRepair UG has been granted the worldwide first 'Orphan Drug Designations' (ODD) by the European Commission / European Medicines Agency (EMA) and the Paediatric Investigation Plan (PIP) for the pivotal trial has been approved. The ODD guarantees market exclusivity in all EU member states for 12 years upon market authorisation. "We are particularly grateful to Olaf Bolwerk, Johan Nijboer, and Dr. Alexander van der Speld who masterminded the transaction to fund our way towards market authorisation", Prof. Dr. Arne Jensen stresses and continues, "All our personal, scientific, clinical, and philanthropic efforts serve the ultimate goal - to combat cerebral palsy, the most common disability in childhood - and stop CP in children!"About BrainRepair UG
BrainRepair UG is a clinical stage start-up developing cutting edge stem cell treatments based on human cord blood for a wide range of indications related to brain injury in children BrainRepair UG is the first Biotech company worldwide whose stem cell products have been awarded 'Orphan Medicinal Product Designations' for the treatment of brain injury in newborn infants (PVL, NE) by the European Commission / European Medicines Agency, EMA. BrainRepair's Headquarter is in Bochum, Germany. You may visit the website at https://brainrepair.eu/ for more information.
Links:
Jensen A. Cerebral palsy - brain repair with stem cells. J Perinat Med. 2022 Dec 12. doi: 10.1515/jpm-2022-0505. Epub ahead of print. PMID: 36503655.
https://www.degruyter.com/document/doi/10.1515/jpm-2022-0505/html
Jensen A. Autologous Cord Blood Therapy for Infantile Cerebral Palsy: From Bench to Bedside, Obstet Gynecol Int vol.2014,12p;
https://www.hindawi.com/journals/ogi/2014/976321/
Jensen A, Hamelmann E. First Autologous Cord Blood Therapy for Pediatric Ischemic Stroke and Cerebral Palsy Caused by Cephalic Molding during Birth: Individual Treatment with
Mononuclear Cells", Case Reports in Transplantation, vol. 2016, Article ID 1717426, 9 pages, 2016.
https://www.hindawi.com/journals/crit/2016/1717426/
Jensen A, Neuhäuser G. Association of weight-length ratio at birth with psychomotor trajectories among preschool-aged children. AJOG Glob Rep. 2022 Oct 2;2(4):100115. doi: 10.1016/j.xagr.2022.100115.
PMID: 36275404; PMCID: PMC9579794.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9579794/
Jensen A, Holmer B. "White Matter Damage in 4,725 Term-Born Infants Is Determined by
Head Circumference at Birth: The Missing Link," Obstetrics and Gynecology
International, vol. 2018, Article ID 2120835, 12 pages, 2018. [doi:10.1155/2018/2120835
https://www.hindawi.com/journals/ogi/2018/2120835/]
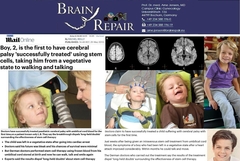
Figure: The first documented trial on transplantation of own (autologous) human cord blood stem cells after global braindamage caused by cardiac arrest (>25 mins) was performed as individual treatment of a boy 2.8 years of age on January 27, 2009 in Bochum, Germany.
GET IN TOUCH
Prof. Dr. med. Arne Jensen, MD, MBA
BrainRepair UG (haftungsbeschränkt)
+49 234 588 196-0
https://brainrepair.eu
You can see the original version and more on PRLeap here: http://www.prleap.com/pr/289025/breakthrough-eur-50-million-investment-for-start-up-brainrepair-ug-pivotal-trial-on-stem-cell-treatment-for-brain-damage-in




