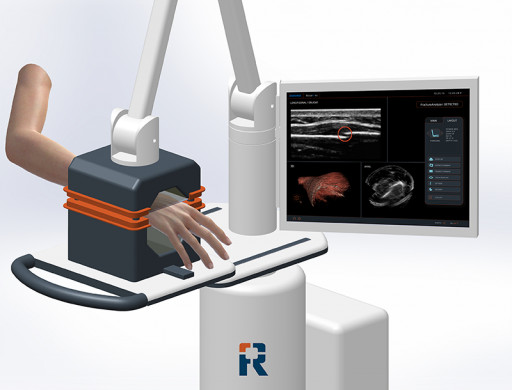
Accuro XV
The Accuro XV is a portable, computer-aided, 3D fracture detection and diagnosis system for rapid, radiation-free, bedside fracture triage.
CHARLOTTESVILLE, Va. - October 26, 2021 - (Newswire.com)
RIVANNA®, developers of imaging-based medical solutions, announced that they have received a contract from the Biomedical Advanced Research and Development Authority (BARDA), a division of the Office of the Assistant Secretary for Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS), for the design and development of a computer-aided 3D fracture detection and diagnosis product, called Accuro® XV. Under the contract 75A50121C00035, HHS/ASPR/BARDA will provide $11.6 million over 24 months with options for additional funding for supporting further development up to $65 million.
The project's overarching objective is to achieve a market-ready, clinically proven and FDA-cleared Accuro XV product for rapid, radiation-free fracture detection and aid triage in emergency medicine; a critical medical countermeasure to ensure timely triage for reduction of morbidity and mortality in mass-casualty blast trauma incidents, for which no field-deployable equipment for extremity fractures currently exists.
The Accuro XV is a portable medical system building on novel technologies that underpin RIVANNA's extensive patent portfolio, including BoneEnhance® Multi-Frequency Image Reconstruction, which optimizes ultrasound for the visualization of bony versus soft-tissue anatomy. Supporting this image reconstruction technology is Multi-Probe Multi-Angle BoneVision™, an automated image-acquisition technique based on a series of novel three-dimensional ultrasound-based bone-imaging technologies that increases angular image sensitivity to bone surfaces. BoneVision provides precisely captured bony anatomical structures within a large field of view and demonstrated performance detecting small fractures with high sensitivity and specificity.
By automating both the image acquisition and image interpretation, Accuro XV will also support just-in-time training, ideal for mass-casualty incidents; the ease of use afforded by the automation will reduce user dependence and optimize the success of clinical evaluations for adoption in hospital and emergency room (ER) settings.
Will Mauldin, PhD, co-founder, and CEO of RIVANNA, commented, "We are pleased to receive this award from BARDA. With attainment of targeted product performance specifications, the clinical and economic value of Accuro XV is expected to result in an inevitable shift away from the current state of X-ray overutilization towards a radiation-free, bedside triage standard of care."
About RIVANNA
RIVANNA® is elevating global standards of care through the development and commercialization of world-first imaging-based medical technologies, including BoneVision™ and BoneEnhance®, which optimize ultrasound image acquisition and visualization to provide radiation-free alternatives to X-ray-based imaging modalities. When paired with SpineNav3D™ AI-Enabled Image Recognition technology, these products offer comprehensive clinician-assistance solutions that improve decision-making, clinician workflows, health outcomes and patient satisfaction. RIVANNA is privately held and operates an FDA-registered and ISO 13485:2016 certified manufacturing facility where it produces the Accuro® product line and related medical equipment and components. For more information, visit rivannamedical.com.
Media Contact: Vicki Brothers | vbrothers@rivannamedical.com
Press Release Service by Newswire.com
Original Source: RIVANNA announces award from U.S. government to develop Accuro Platform for rapid triage of blast-related injuries