Articles published by Edgewise Therapeutics

Edgewise Therapeutics Reports Third Quarter 2023 Financial Results and Recent Business Highlights
November 09, 2023
Via Business Wire
Tickers
EWTX

Edgewise Therapeutics to Present at Upcoming Investor Conferences
November 08, 2023
Via Business Wire
Tickers
EWTX

Edgewise to Present Data on EDG-7500 at the American Heart Association’s Scientific Sessions 2023
November 01, 2023
Via Business Wire
Tickers
EWTX





Edgewise Therapeutics Reports Second Quarter 2023 Financial Results and Recent Business Highlights
August 10, 2023
Via Business Wire
Tickers
EWTX
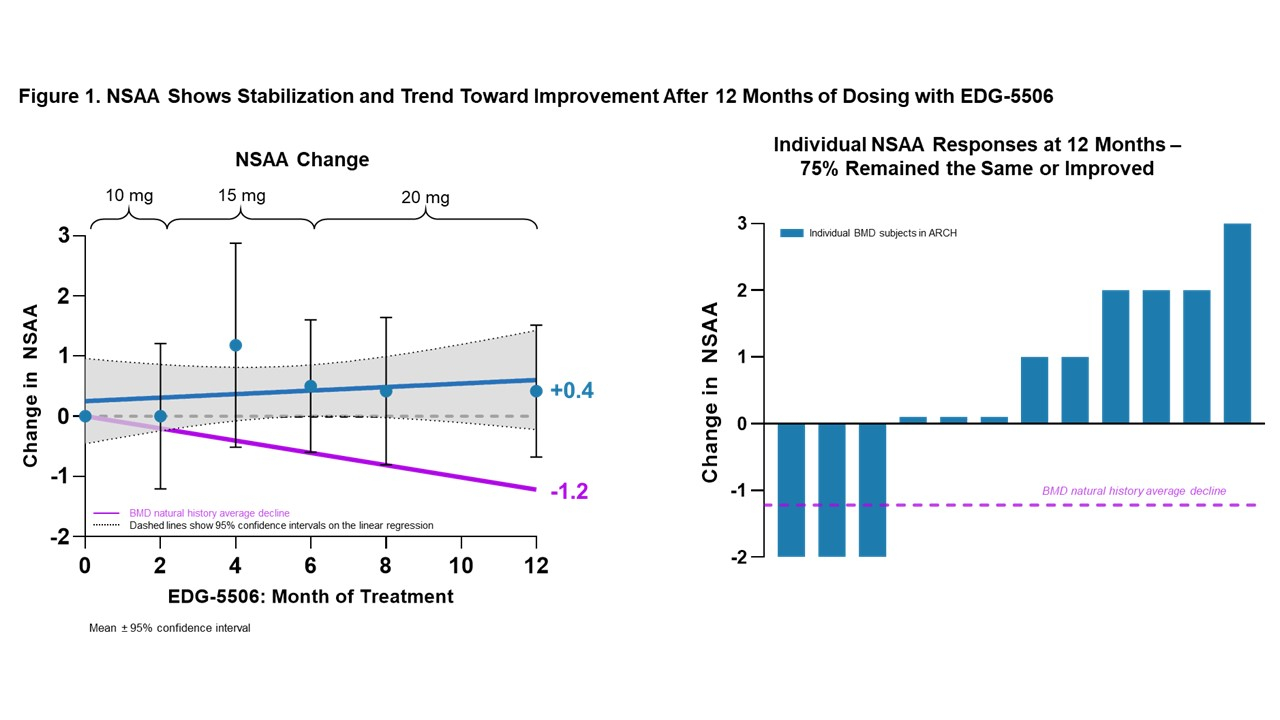

Via Business Wire
Tickers
EWTX


Via Business Wire
Tickers
EWTX

Via Business Wire
Tickers
EWTX

Via Business Wire
Tickers
EWTX



Edgewise Therapeutics to Present at the Cowen 43rd Annual Health Care Conference on March 8, 2023
March 01, 2023
Via Business Wire
Tickers
EWTX



Edgewise Therapeutics to Present at the SVB Securities Global Biopharma Conference on February 14, 2023
February 07, 2023
Via Business Wire
Tickers
EWTX

Edgewise Therapeutics to Present at the 41st Annual J.P. Morgan Healthcare Conference on January 10, 2023
January 04, 2023
Via Business Wire
Tickers
EWTX

Edgewise Therapeutics to Showcase Novel Cardiac Program at Virtual Investor Event on Tuesday, January 3, 2023
December 21, 2022
Via Business Wire
Tickers
EWTX

Via Business Wire
Tickers
EWTX

Edgewise Therapeutics to Present at Upcoming Investor Conferences
November 09, 2022
Via Business Wire
Tickers
EWTX

Edgewise Therapeutics Reports Third Quarter 2022 Financial Results
November 03, 2022
Via Business Wire
Tickers
EWTX

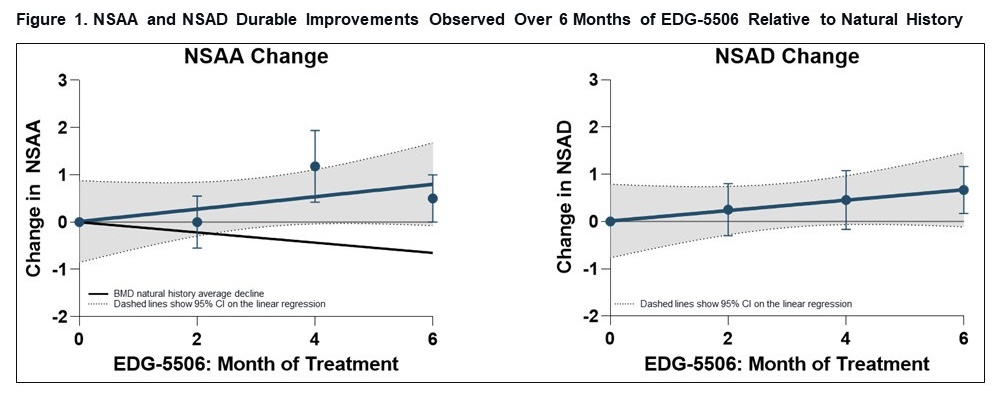


Edgewise Therapeutics Announces Pricing of Upsized Public Offering of Common Stock
September 13, 2022
Via Business Wire
Tickers
EWTX

Edgewise Therapeutics Announces Proposed Offering of Common Stock
September 13, 2022
Via Business Wire
Tickers
EWTX
Stock Quote API & Stock News API supplied by www.cloudquote.io
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
Quotes delayed at least 20 minutes.
By accessing this page, you agree to the Privacy Policy and Terms Of Service.
© 2025 FinancialContent. All rights reserved.